Abstract
Introduction:
Patients with genomic aberrations del(11q), del(17p), and mutated IGHV in chronic lymphocytic leukemia (CLL) are at the highest risk for poor outcomes including shorter time to initial treatment, non-response to therapy (refractory disease), relapse, reduced progression-free survival (PFS) and overall survival (OS). Response to CLL therapy has typically been defined as complete remission (CR) or partial remission (PR), although recent evidence demonstrates that minimal residual disease (MRD) status is a more comprehensive indicator of treatment response. MRD negativity (MRDn) versus positivity (MRDp) has been associated with better PFS and OS independent of treatment response. There is little evidence on how economic outcomes such as lifetime costs vary by these response categories. The objective of this study was to quantify the difference in CLL-related lifetime costs for patients across four response categories: MRDn/CR, MRDn/PR, MRDp/CR, and MRDp/PR.
Methods:
A 19-state cohort Markov model to estimate the clinical outcomes and direct medical costs of CLL for patients achieving the four response categories of interest was created. A cohort of newly diagnosed CLL patients were simulated in quarter-year cycles through disease progression until all patients in the cohort died. Transition probabilities through diagnosis, treatment response, relapse, and death were estimated using data from clinical trials and the published literature. Mortality was estimated using US Life Table data from 2010 and CLL-specific mortality estimates from the literature. Patients were stratified into high risk (del(11q), del(17p), and unmutated IgVH) and low risk (all other patients) groups in the model. Treatment costs and subsequent response rates were modeled after 2017 NCCN guidelines and current clinical practices. All cost parameters were converted to 2016 USD and future costs were discounted at a rate of 3%.
Results:
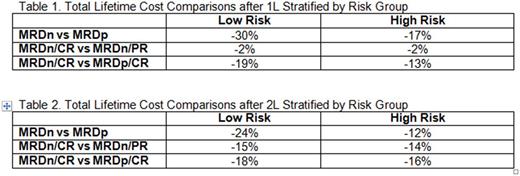
The model estimates that lifetime CLL-related costs range from $140,250 - $368,844 per patient and that patients achieving MRDn/CR after first line (1L) therapy experience the lowest lifetime costs. Tables 1 and 2 show the incremental change in costs across various response categories after 1L and second line (2L) therapy. The most substantial cost savings were associated with MRDn responses compared to MRDp responses. Patients achieving MRDn in 1L therapy, on average, incurred 30% lower costs than those who were MRDp (Table 1). This effect was also observed among CR patients in which, patients achieving MRDn/CR in 1L therapy, on average, had 19% lower costs than patients achieving MRDp/CR. The results are consistent in 2L therapy (Table 2).
Conclusion:
MRDn is associated with reduced CLL-related lifetime costs regardless of clinical response (CR or PR). Previous research has indicated that MRD status is the most significant predictor of PFS and OS and this study implies that patients achieving MRDn will also incur less lifetime costs related to CLL.
Sail: AbbVie Inc: Employment, Equity Ownership, Other: I am an employee of AbbVie, Inc and may own AbbVie and/or Abbott stock. Ward: Precision Health Economics: Employment, Other: research funding from AbbVie Inc. Iyenger: AbbVie Inc: Employment, Equity Ownership, Other: I am an employee of AbbVie, Inc and may own AbbVie and/or Abbott stock. Silverstein: Precision Health Economics: Employment, Other: research funding from AbbVie Inc. Espinosa: Precision Health Economics: Employment, Other: research funding from AbbVie Inc. Keim: AbbVie Inc.: Employment, Equity Ownership, Other: I am an employee of AbbVie, Inc and may own AbbVie and/or Abbott stock. Meissner: AbbVie Inc.: Employment, Equity Ownership, Other: I am an employee of AbbVie, Inc and may own AbbVie and/or Abbott stock. Frasco: Precision Health Economics: Employment, Other: research funding from AbbVie Inc.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal